Research associates from MIT, UT collaborate to develop new lithium-ion battery, increase its capacity
April 6, 2021
Researchers at the Massachusetts Institute of Technology and UT-Austin collaborated in a March 25 study to increase the capabilities of lithium-ion batteries, which are often used in portable devices such as phones, laptops and electrical vehicles.
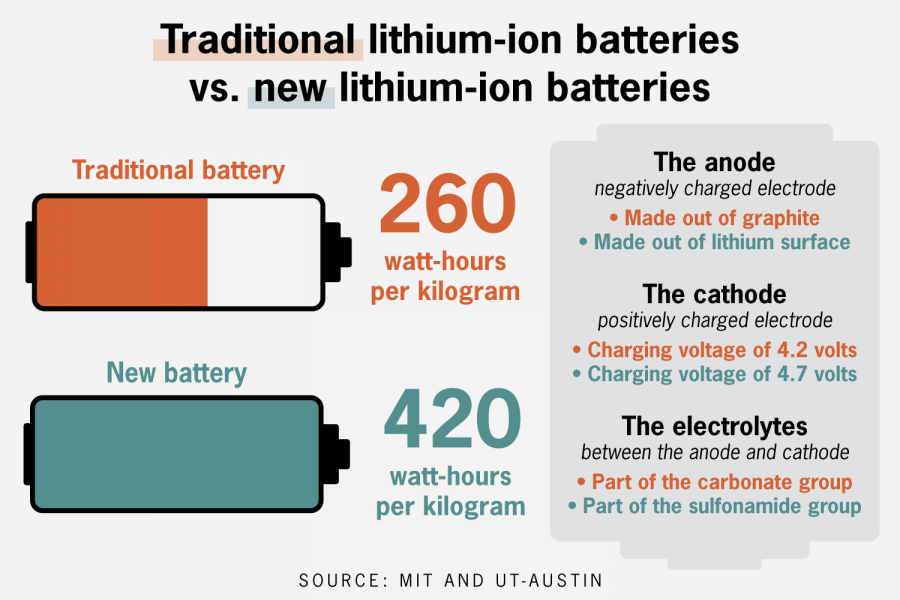
The team created a new lithium-ion battery that can store twice the power of typical lithium-ion batteries, MIT postdoctoral associate Weijiang Xue said.
Xue said he hoped to increase the energy density of batteries, which is how much energy a battery can carry compared to its weight. He replaced the graphite used in the negative end of a battery, the anode, with lithium metal, and increased the voltage of the positive end, the cathode.
“Lithium metal is very dangerous, but … its (energy) capacity is more than ten times of the graphite,” Xue said.
MIT chemistry professor Jeremiah Johnson said there’s an issue with this approach. He said an unwanted chemical reaction occurs between the two ends of the battery and the chemical medium between them, called the electrolyte, which prevents the battery from working properly.
“Lithium is such a reactive material — it’s (on the) far left of the periodic table, which means that it’s a very strong reducing agent; it likes to give electrons to things,” Johnson said. “When it does that, a lot of the classic electrolytes … aren’t stable. They can’t survive that.”
In order to develop the new battery, Xue said he worked with Johnson’s lab. He said Johnson’s team had previously developed a novel electrolyte that resists oxidation, the loss of electrons, and harsh reactions for a different type of battery.
Xue said the novel electrolyte is made of a sulfonamide group, a chemical group found in sulfa drugs, which are used to treat bacterial infections. Johnson said conventional electrolytes — which are made out of carbonate, a common compound found in chalk and limestone — are not stable in high voltage and in the presence of lithium metal.
Xue said the cathodes would often crack because of how unstable they are with conventional carbonate electrolytes. The new electrolyte prevents the cracking behavior, allowing for the development of a new lithium-ion battery, Xue said.
“The (new electrolyte) has very good oxidation resistance,” Xue said. “So that means when we charge the cathode with a very high voltage, (the new electrolyte) can be very stable with the cathode surface.”
Johnson said the new battery’s larger capacity could allow for longer range for electric vehicles and shorter charging times for mobile devices.
UT research associate Yutao Li said his role on the project was to find out if the new battery had any improvements compared to conventional batteries. He said the new battery could be very applicable if MIT increases the battery’s cycle life, the amount of times it can be fully discharged and recharged, and reduces the cost of the electrolyte.
Johnson said the team is working with a contract facility and multiple battery companies to create more of the new electrolyte to test on larger batteries.
“If it works as well in a company’s hands as ours, it could actually be the thing that’s in all of our phones,” Johnson said. “It’s kind of crazy, but it actually has that level of potential.”
Editor’s Note: This article first appeared in the April 6 issue of The Daily Texan.