By studying the interactions of a useful but toxic molecule with applications in freezing transplant organs, a team of UT researchers is laying the foundation for safer organ storage and transport.
Ice crystals that result from current attempts to freeze organs before transplantation can destroy the tissue, according to Carlos Baiz, chemistry assistant professor. But cryoprotectants, molecules such as dimethyl sulfoxide (DMSO) that prevent ice crystal formation, are currently too toxic to be used.
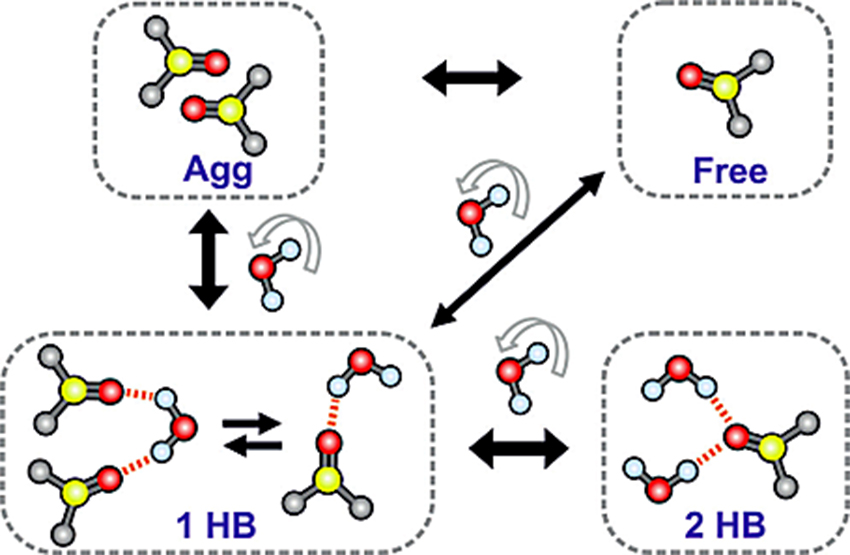
Scientists in the UT Chemistry Department may have found one of the puzzle pieces needed to make freezing organs more successful. The team examined the hydrogen bonds between DMSO and water molecules to understand how DMSO prevents the formation of ice crystals in research published in journal Angewandte Chemie.
“Even though it’s a very simple question [of] how many hydrogen bonds does DMSO make with water, it’s actually really complicated, since molecules move very quickly,” Baiz said.
Baiz said the team used infrared absorption spectroscopy to measure the vibrations of the double bond between sulfur and oxygen atoms in DMSO. The oxygen atom forms hydrogen bonds, intermolecular interactions between hydrogen atoms from one molecule and atoms in neighboring molecules, with other DMSO molecules and water. This shows through spectrometry as a change in the frequency of bond vibrations.
Using spectroscopy allowed the researchers to capture an instantaneous snapshot of the sample, unlike other techniques that take a long time and result in a blurry picture, Baiz said.
The study found that at low concentrations, DMSO forms two hydrogen bonds with water; at medium concentrations, DMSO forms only one hydrogen bond with water; at very high concentrations, DMSO associates and aggregates with other DMSO molecules.
Research Associate Kwang-Im Oh said this method allowed the team to look closer at DMSO bonding than other methods.
“The possible configurations of hydrogen bond between water and DMSO molecules are fairly well-predicted using traditional simulations,” Oh said. “However, specific hydrogen bond populations and vibrational frequencies of DMSO-water interactions have not been resolved from computations.”
Baiz said the idea stemmed from a Plan II research class in the Psychology Department.
“Because this field is still outside of mainstream science, with a little bit of research, we can make a big contribution,” Baiz said. “We didn’t have a lot of literature to rely on, so we had to develop most of the technique and analysis ourselves.”
Their next step is to investigate how DMSO interacts with more complex molecules to lower its toxicity, Baiz said.
“If we can prevent ice formation, we can increase the survivability of cells,” Baiz said.
Previous studies have indicated that adding formamide, which neutralizes toxicity, could make DMSO less toxic to cells, said undergraduate researcher Kavya Rajesh.
Rajesh said the next step for the group will be to use this new information about how DMSO interacts with other molecules to make it safer for living organisms.
”The plan is to study how DMSO interacts with water and formamide, and see if formamide affects the way DMSO interacts with water and biological systems,” Rajesh said.