New COVID-19 vaccine developed with UT technology enters human trials, beneficial for low-income, intermediate-income countries
April 15, 2021
A cheaper and potentially more potent COVID-19 vaccine, which utilizes scientific advancements pioneered by a UT researcher, entered human clinical trials this month in Vietnam, Thailand, Brazil and Mexico.
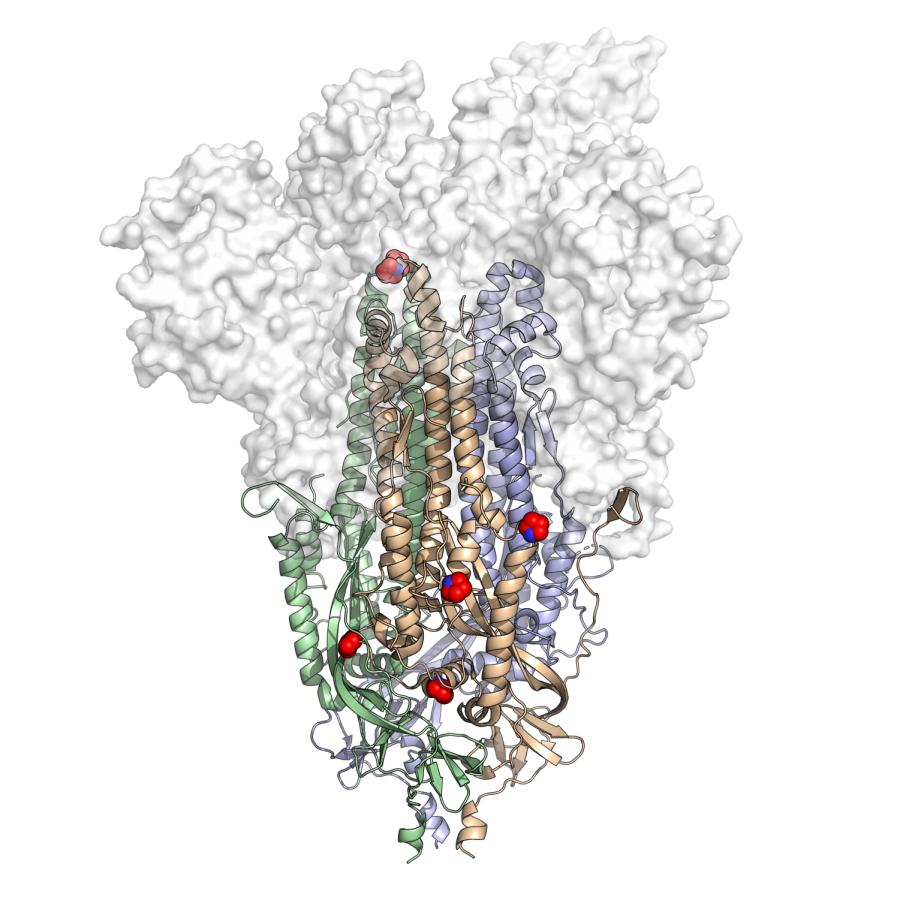
The new vaccine, NDV-HXP-S, relies on a modification of a spike protein, the part that allows viruses to insert their genetic material into our cells, called HexaPro. The McLellan Lab, which is led by Jason McLellan, an associate professor of molecular biosciences at UT, engineered the protein.
Jory Goldsmith, a graduate student at the lab, said preliminary experiments show that vaccines containing HexaPro are more potent than those with the previous stabilized spike protein because human antibodies recognize and respond to Hexapro better since the spike protein is less prone to shifting shapes.
“There is at least … some published evidence now that HexaPro vaccines are just generally better than vaccines that only have the two (mutations),” Goldsmith said
Goldsmith said the lab found that the spike proteins on the surface of coronaviruses are unstable, making vaccine development more difficult.
“We realized we needed to make this protein more stable in order to make it a better vaccine, (and) make it easier to produce vaccines in larger quantities,” Goldsmith said.
After altering and testing 100 versions of the spike protein, the researchers created HexaPro by introducing six mutations that created a more stable spike protein. The older version of the modified spike protein only possessed two mutations, and labs used it to produce currently available vaccines, including Moderna, Pfizer and Johnson & Johnson.
The new vaccine is grown in chicken eggs, a common method for creating flu vaccines, making it more affordable and allowing countries to produce their own vaccine rather than importing it from elsewhere.
Administrators can store the NDV-HXP-S vaccine between two and eight degrees Celsius for at least a week, said Ching-Lin Hsieh, a postdoctoral researcher at the lab.
Countries can store Pfizer vaccine vials between two and eight degrees Celcius for up to five days before mixing, Moderna vials can be stored at the same temperature for up to 30 days before being administered, and Johnson & Johnson vials can be stored at the same temperature until its expiration date, according to the Centers for Disease Control and Prevention.
“In terms of distributing the vaccine around the world to the low-income (and) intermediate-income countries, this type of vaccine has a huge advantage,” Hsieh said.