UT researchers create new method to capture lithium that could help meet demand for electric cars
September 26, 2021
UT doctoral students are part of a team developing a new method of lithium extraction, which could help meet high demand for the element and reduce its cost.
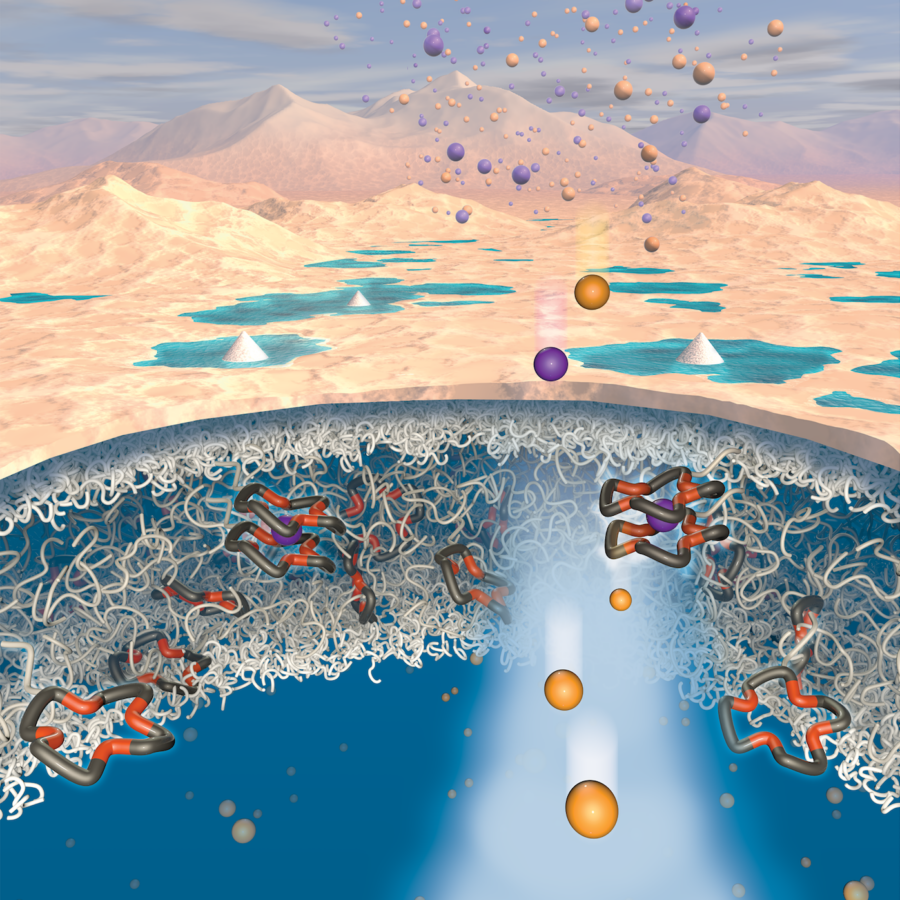
Lithium, which is used to power electronics, appliances, electric vehicles and other everyday items, can be found almost anywhere in the world, but the extraction process is slow and inefficient, according to Everett Zofchak, part of the research team that published their findings Sept. 7.
Zofchak, a chemical engineering doctoral student, said the research team developed a new polymeric membrane that can separate lithium salts from sodium salts and simplify the extraction process.
Zofchak said current conventional membranes lack the ability to differentiate between similar species such as lithium and sodium.
“If you can use a membrane-based process, you could drastically improve the efficiency and the production of lithium, which would help us with pushing towards things like electric vehicles,” Zofchak said.
An efficient extraction process can increase the resources available for use in electric cars, according to Zofchak.
“The reason why we really wanted to do this (study) is that current lithium production processes will not be able to scale in the future to meet demands that are projected for things like ion batteries for electric cars,” Zofchak said.
Rahul Sujanani, a chemical engineering doctoral student and co-author of the study, said the project addresses the current limitations to lithium extraction and potential ways to collect lithium more efficiently.
“In our recent work, we incorporated ion specific sites into polymers to develop a material that can permeate lithium salts faster than sodium salts,” Sujanani said in an email. “The results from our fundamental studies provide important design rules for developing membranes that are specific for one solute, which would be a crucial advance for applying membrane technology to new separation applications, including lithium recovery.”
Zofchak said lithium-ion batteries can store a lot of charge with little material, so the next step for the researchers is to test their membrane in a real setting as opposed to online prototypes.
“As far as what this technology does for electric vehicles, there’s still a lot of work that needs to be done before you can do direct capture of lithium,” Zofchak said. “It’s a really challenging thing and I don’t think that this technology is the end all be all, things need to improve quite a bit. But I think that we’re headed in the right direction.”