New drug found by UT study to outperform standard stroke medication
October 9, 2022
Tenecteplase: a new-wave drug originally used to treat heart attacks outperforms a standard medication, alteplase, for treating ischemic strokes, according to a new study released by the American Stroke Association’s journal.
Steven Warach, a neurology professor and director of the Stroke Program for Dell Medical School and Ascension Texas, led the cohort study that revealed tenecteplase’s quicker administration time, increased favorable health outcomes and cheaper hospital cost.
Because strokes and heart attacks both deal with blood clots, researchers thought tenecteplase could be applied to stroke patients. Starting in Sept. 2019, 10 Ascension hospital networks carried out the study for 15 months by giving stroke patients the tenecteplase shot.
The FDA originally approved tenecteplase for treating heart attacks, but the researchers in this study explored tenecteplase’s impact on stroke patients. Tenecteplase is administered through a shot, which improves time efficiency compared to alteplase, which is administered through IV.
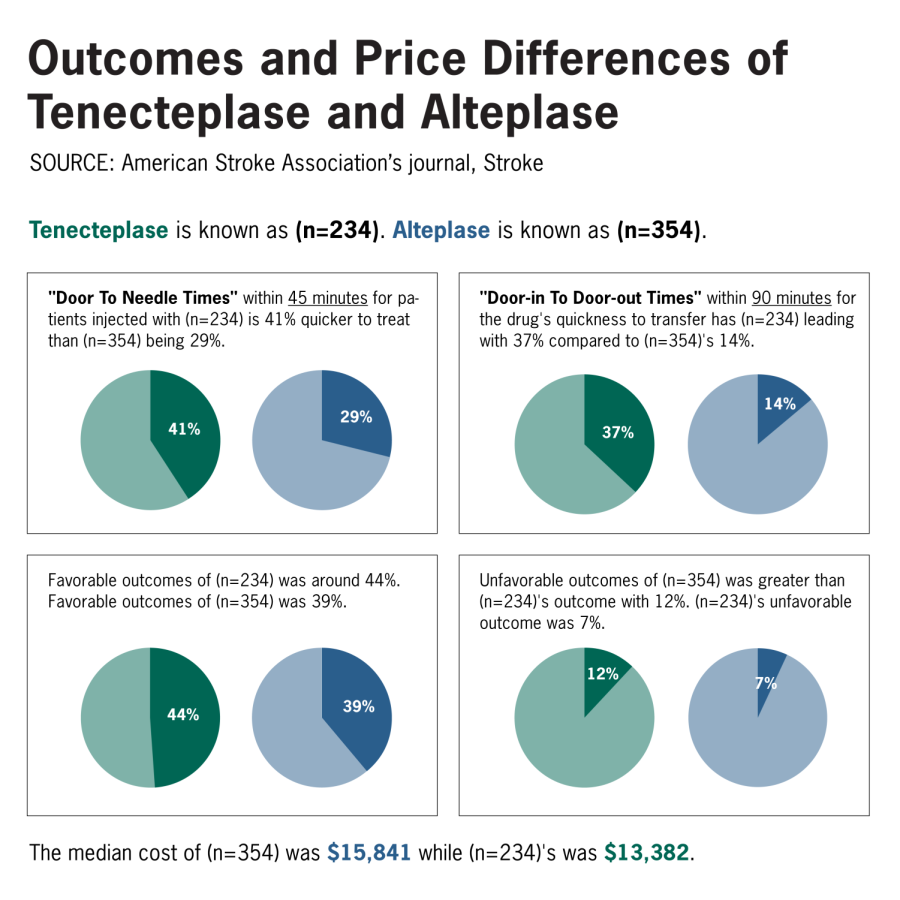
The administration time from once a patient arrives at the hospital to when they are transferred to a higher level hospital, within at least 90 minutes, had tenecteplase leading with 37% compared to 14% from alteplase. Additionally, the time between the patient’s arrival and treatment administration within 45 minutes had tenecteplase leading with 41% compared to 29% from alteplase.
“What we’re hoping to confirm and we actually did was that because of (tenecteplase’s) properties of being a quick 10-second injection versus a one-hour infusion, we would be able to initiate clot-busting therapy for stroke quicker,” Warach said.
Although tenecteplase may be proven to treat stroke patients with better outcomes, Warach said they found tenecteplase to be “non-inferior” or “at least as good as” alteplase. According to the study, patients who received tenecteplase were discharged home and walking independently at a better rate than patients who received alteplase. Tenecteplase patients also had fewer negative side effects, including brain hemorrhage, than patients who received alteplase.
“Tenecteplase has greater value in outcomes, and what that means is improved outcomes at a better cost,” said David Paydarfar, co-author of the study and professor and chair of the department of neurology.
Another improvement of tenecteplase is the cost, Paydarfar said. The differentials could help save money in the long run.
“The drug itself is less expensive right now,” Warach said. “It’s about $2,500 to $2,000 less per dose of the tenecteplase versus the alteplase.”
Though tenecteplase has yet to be FDA approved for treating strokes, the switch to tenecteplase has already begun with the 10 network Ascension hospitals and Warach has started to see the adoption in other hospitals around the state.
Paydarfar related the study’s application of tenecteplase for stroke treatment to birth control, which is FDA approved for preventing pregnancies, but not for menstrual regulation or to prevent headaches. However, doctors are still able to prescribe birth control for these reasons.
“UT Austin Dell Medical School really put their foot on the accelerator to get tenecteplase to people who can use it and who can benefit from it,” Paydarfar said. “I think it’s a very good example of how the med school is innovative.”